
Bringing women’s healthcare out of the dark ages.
Applying brain-based technologies to the bodies science overlooked.
A new perspective starts in the brain, and results in real care.
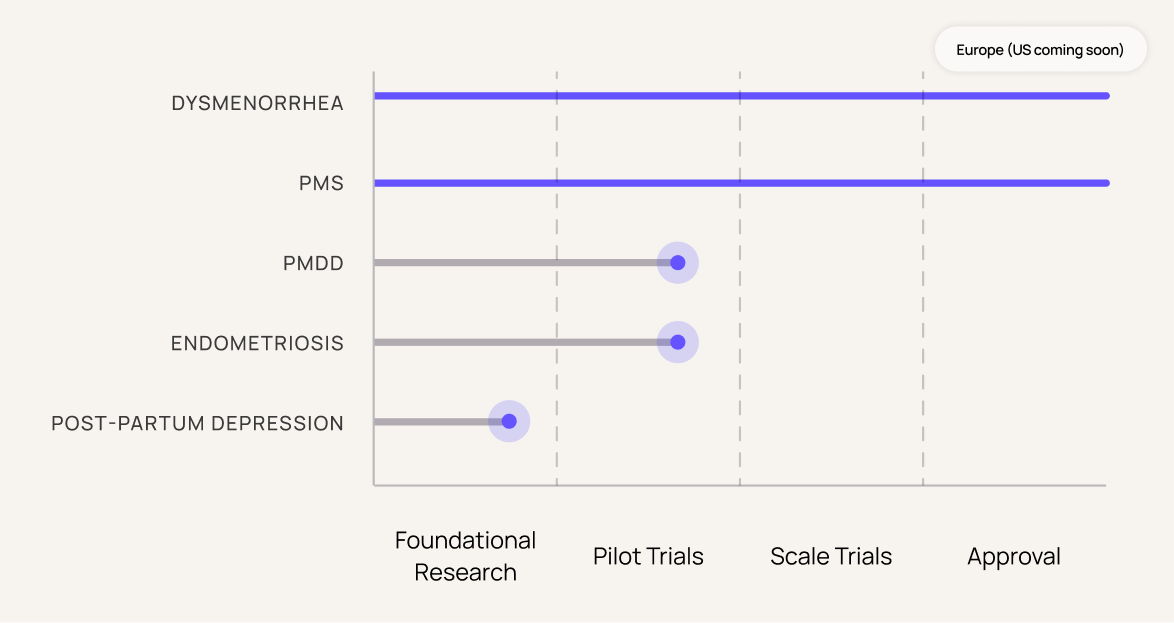
Research Pipeline
Most women’s health conditions can be targeted through brain-based approaches. Even when the cause lies outside the brain, these approaches offer lasting symptom relief -- increasing patient dignity and control while deeper research continues.
Our flagship solution, Nettle, has been subject to multiple clinical trials, with a comprehensive pipeline of follow-up studies planned.
Women's health has been underfunded and overlooked.
We use neuroscience, clinical technology and thoughtful design to create tools that help you feel more clear, steady and in control. From brain fog, to burn out and beyond.
The system's catching up.
Women's health isn't niche. It's necessary.
Pilots and Trials
We’re working with clinical partners, researchers and regulators around the world on a growing portfolio of clinical trials and healthcare system pilots. Below are just a few in progress or complete.
Research background
Our technologies are grounded in more than three decades of foundational research. A selection of papers, some written by our team and our advisors, and others from the broader community, can be found below.



