






Studied, designed and supported by teams from






Studied, designed and supported by teams from
Nettle™
Medical Wearable for Menstrual Pain and Mood Relief
Nettle™ is not available in US
You're browsing our US store, where Nettle™ is not available. Discover Lutea™, based on the same award-winning technology, and available in your region.

Stay up to date with Nettle™
See what our users are saying



Why the brain
89% of our users report using fewer medications
All statistics taken from the Six Month Nettle User Survey (2025)
0%
of users report "fewer sick days or interruptions to daily life"*
0.0
fewer GP or specialist visits reported on average per user per year
0%
of all users reduced their use of hormonal treatments.
Using Nettle™ is easy.
It's not random. It's a rhythm.
Even if its complex or irregular, your cycle has a pattern you can work with, not just endure. Build a Nettle™ schedule that is flexible enough for real life yet precise enough to support you when it matters most.

It's not random. It's a rhythm.
Even if its complex or irregular, your cycle has a pattern you can work with, not just endure. Build a Nettle™ schedule that is flexible enough for real life yet precise enough to support you when it matters most.


Session scheduled
21 min ago
Tap to start.

Great progress
11 min ago
You're halfway through your session.

Session complete
Now
Log how you feel to improve your schedule.
Be notified when you need it
Track your experiences in the Samphire app, and be notified when Nettle™ needs to be used.
Run sessions
Setup takes under a minute, and sessions run for twenty minutes.

Live life
Once your session starts, you are free to carry on—rest, work, or move through your day as usual. Nettle™ works quietly in the background, supporting you throughout your cycle.

Monitor and adjust over time
Track patterns across cycles and learn what works best for your body. Support your cycle with confidence with Lutea™
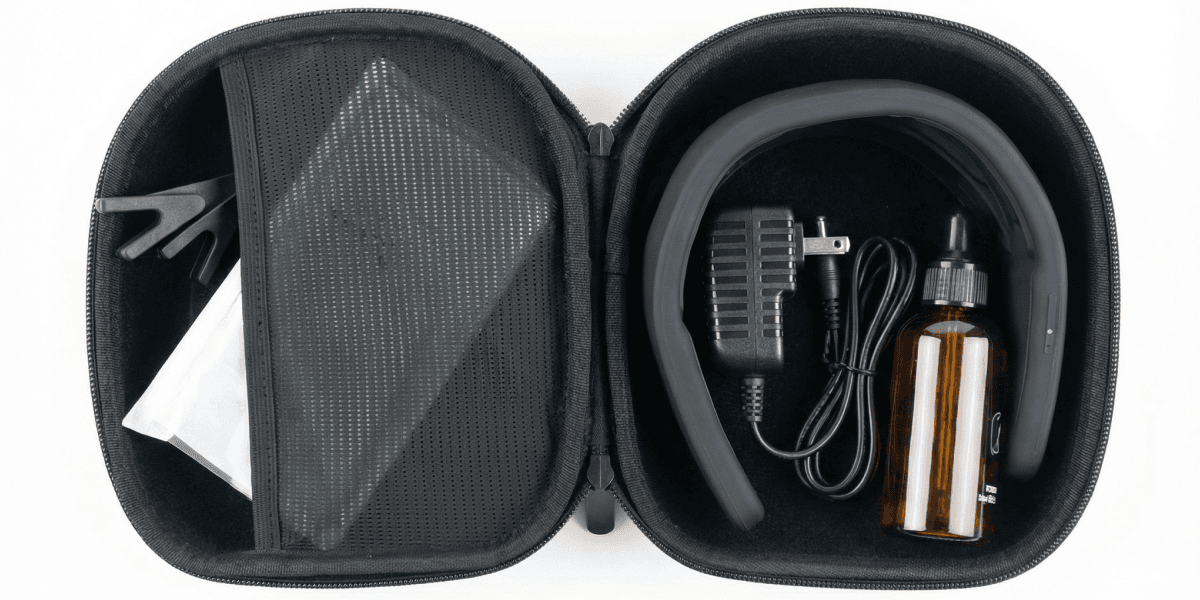
Everything you need
Nettle™ comes with everything you need for stress-free stimulation.

Protect your Nettle™ when you're on the go.




Why the brain
Clinically tested for real-life support.
Nettle™ uses neuroscience, clinically tested technology and thoughtful design to create tools that help you feel more in control - improving focus, easing burnout, supporting mood and reducing brain fog.

It’s all in your brain.
Mood, pain, motivation - they start and end in the brain.

It’s not random. It’s a rhythm.
Even if its complex or irregular, your cycle has a pattern you can work with, not just endure.

Clinically tested real-life support.
Nettle™ neuroscience and smart design to help you feel clearer, steadier, and in control.
Simple, effective and safe.
How to use Nettle™
Designed from the ground up to be easy to use with no severe side effects, Nettle™ easily integrates into your life - no matter what your day looks like.

Schedule
Get notified when you need it

Setup
Be ready to go in under two minutes

Integrate
Use Nettle™ during other activities

Monitor
See your progress
Brain, body, mind – all working together.
Decades of research show that the brain can rewire itself - with the right kind of stimulation. Pair Nettle™’s cutting edge science with holistic care in the Samphire app. Working with your cycle, not against it, you build a foundation for relief.

Mood Swings
Nettle™ rebalances activity in the brain’s prefrontal cortex, helping calm fight-or-flight responses.

Integrated care
Nettle™ integrates with Samphire, giving you personalised guidance across your entire cycle.


Pain Relief
Nettle™ retrains the brain’s response to pain by modulating overactive pathways - teaching your system to react differently.

Cycle resilience
Each time you use Nettle™, you’re creating adaptive brain responses to boost your overall reslience.


Mood Swings
Nettle™ rebalances activity in the brain’s prefrontal cortex, helping calm fight-or-flight responses.

Integrated care
Nettle™ integrates with Samphire, giving you personalised guidance across your entire cycle.

Pain Relief
Nettle™ retrains the brain’s response to pain by modulating overactive pathways - teaching your system to react differently.

Cycle resilience
Each time you use Nettle™, you’re creating adaptive brain responses to boost your overall reslience.
94% of users feel better in just three months.*
We invest in rigorous science, conducting randomized, placebo-controlled, double-blind clinical trials to ensure the safety and effectiveness of Nettle's™ technology.




97%
of users find Nettle™ easy to integrate into their lives.
67%
noticed mood improvements within one cycle.
72%
experienced reduced pain within one cycle.
93%
felt relief within three cycles of personalized use.
What we help with
Explore our perspectives on women’s health below.
Too often, systems treat symptoms in isolation. Samphire’s solutions work across the brain, body and cycle - connecting the dots between pain, mood, focus, hormones and more.
Our Blogs














