
Pain
•
2025-08-24
How painkillers work - and why they work differently for menstrual, endometriosis, and widespread pain
By Kate Ferguson, Chief of Staff at Samphire Neuroscience
Painkillers have often been the first thing I reach for when cramps or flare-ups hit - and as a woman with endometriosis, that’s been a constant in my life. For years, it felt like the only option: swallow a pill, wait for relief, and hope for the best. Sometimes it worked. Other times, it barely touched the sides.
It turns out, neuroscience can explain why. From how your brain processes pain to the different ways medications target (or miss) the real source, understanding this science can help you choose better, more effective relief - and see why brain-based solutions are giving people like me more options.
How the brain processes pain signals
Pain begins in the body - say, in the uterus when prostaglandins, hormone-like chemicals that trigger muscle contractions and inflammation, surge - but it is constructed in the brain.
Signals start in nociceptors (specialized nerve endings that detect potential harm) and travel along sensory nerve fibres to the dorsal horn of the spinal cord, a hub where incoming pain messages are processed. From there, they move upward through the spinothalamic tract, the main highway for pain and temperature signals, to the thalamus, a relay station that sends information to other brain areas.
The message then reaches the somatosensory cortex, which maps pain to a specific body location. Other areas shape how pain feels: the insula integrates sensory input with emotion, the anterior cingulate cortex processes the unpleasantness of pain, and the amygdala links pain to emotional memories.
The periaqueductal gray (PAG), a small but powerful structure, can switch on descending pain inhibition - the brain’s built-in system for sending signals down the spinal cord to reduce or block incoming pain messages.
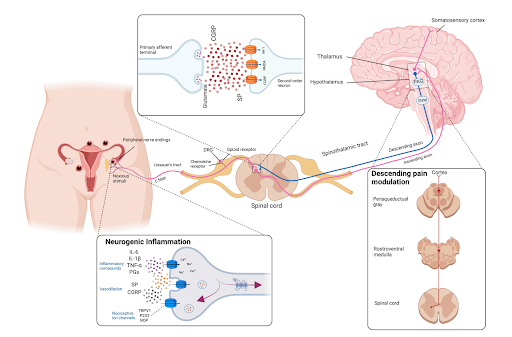
This diagram shows how pain from conditions like endometriosis travels from the uterus to the brain, and how brain regions like the periaqueductal gray (PAG) can modulate it. It also illustrates the chemical messengers - such as prostaglandins, substance P, and glutamate - that play a role in amplifying or calming pain. Adapted from Guan Q., Voltolini Velho R., Sehouli J., Mechsner S. “Endometriosis and Opioid Receptors: Are Opioids a Possible/Promising Treatment for Endometriosis?” International Journal of Molecular Sciences, 2023
In conditions like primary dysmenorrhea (period pain without an underlying medical condition) and endometriosis (where tissue similar to the lining of the uterus grows outside it), brain imaging shows that these networks can be wired differently. Repeated pain can lead to central sensitization - a state where nerve cells in the brain and spinal cord become hyper-responsive, neurotransmitters (chemical messengers that carry signals between nerve cells) become unbalanced, and pain pathways overreact. In this state, glutamate (which increases nerve activity) may be too high, while GABA (which calms nerve activity) may be too low.
How painkillers take on period pain at its source
For menstrual cramps, prostaglandins are the main culprit - chemical messengers that trigger uterine contractions and inflammation. The higher the prostaglandin levels, the stronger and more painful the cramps.
- NSAIDs (like ibuprofen, naproxen, mefenamic acid and diclofenac) block the COX enzymes that produce prostaglandins. This reduces inflammation, eases contractions, and lowers the number of pain signals hitting the brain.
- Acetaminophen (paracetamol) works mostly in the brain and spinal cord to change how pain is perceived. It doesn’t tackle inflammation but can still reduce the “volume” of pain.
- Combination therapy - using both NSAIDs and acetaminophen - can address both the source of pain in the uterus and the way it’s processed in the brain.
How painkillers work for period pain
For menstrual cramps, prostaglandins are the main culprit.
- NSAIDs (non-steroidal anti-inflammatory drugs such as ibuprofen, naproxen, mefenamic acid, and diclofenac) block cyclooxygenase (COX) enzymes, which are needed to produce prostaglandins. This reduces inflammation, reduces uterine contractions, and lowers the number of pain signals reaching the spinal cord. Research also suggests they may act on the central nervous system by reducing prostaglandin levels in the cerebrospinal fluid, calming brain pain networks.
- Acetaminophen (paracetamol) acts mainly in the central nervous system, possibly by blocking a variant of the COX enzyme in the brain and increasing serotonin (a neurotransmitter that helps suppress pain signals) activity in the descending pain inhibition system. While it doesn’t reduce inflammation, it changes how pain is processed.
- Combination therapy - NSAIDs for peripheral inflammation plus acetaminophen for central pain modulation - can be more effective than either drug alone.
Why painkillers work better for some people than others
Painkillers for primary dysmenorrhea
When prostaglandins are the main driver, NSAIDs tend to work well. But even between periods, some people with dysmenorrhea show altered connections between the PAG, thalamus, and cortex. If descending pain inhibition is already underperforming, blocking prostaglandins only addresses part of the pain pathway.
Painkillers for endometriosis and adenomyosis
Endometriosis and adenomyosis (where endometrial tissue grows into the muscle wall of the uterus) often begin with inflammation but can progress to central sensitization. Over time, nerve pathways adapt: glutamate activity increases, GABA activity decreases, and the brain essentially “learns” the pain. This can keep symptoms going even when inflammation is under control. NSAIDs can still help during flares, but fuller relief often comes from therapies targeting brain modulation - like Nettle™, which delivers gentle stimulation to areas such as the insula to raise the pain threshold.
Painkillers for fibromyalgia and widespread pain conditions
Fibromyalgia and other whole-body pain syndromes are less about prostaglandins and more about neurotransmitter imbalances. Research shows higher levels of glutamate and substance P (a chemical messenger that boosts pain signal transmission) and lower levels of GABA, serotonin, and norepinephrine (a neurotransmitter that, like serotonin, helps suppress pain). This imbalance turns the body’s pain “volume dial” up, making even gentle touch painful. NSAIDs rarely help; centrally acting drugs like SNRIs (serotonin-norepinephrine reuptake inhibitors), gabapentinoids, and brain-focused therapies are usually more effective.
Where pain relief is headed next
Chemical painkillers remain important tools, but they’re not one-size-fits-all. For conditions where the brain is the primary amplifier, targeting the source is only part of the picture.
That’s why we created Nettle™ - a non-invasive, drug-free wearable that delivers gentle brain stimulation to modulate pain pathways. For menstrual pain, endometriosis, and central sensitization syndromes, Nettle™ can work with the brain - not just against the body - and may offer relief where traditional pills can’t.
References
Barth, C. et al. (2016) ‘In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle’, Scientific Reports, 6(1), p. 32833.
Becker, S. and Schweinhardt, P. (2012) ‘Dysfunctional Neurotransmitter Systems in Fibromyalgia, Their Role in Central Stress Circuitry and Pharmacological Actions on These Systems’, Pain Research and Treatment, 2012, p. 741746.
Damascelli, M. et al. (2022) ‘Multiple Functional Brain Networks Related to Pain Perception Revealed by fMRI’, Neuroinformatics, 20(1), pp. 155–172.
Grandi, G. et al. (2012) ‘Prevalence of menstrual pain in young women: what is dysmenorrhea?’, Journal of Pain Research, 5, pp. 169–174.
Kumari, *Preeti and Iqbal, M.S.G. and D.R. (no date) ‘A REVIEW ARTICLE ON ANALGESIC AND ANTI-INFLAMMATORY TREATMENTS IN MENSTRUAL CRAMPS’, World Journal of Pharmaceutical and Medical Research, 2023(VOLUME 9, SEPTEMBER ISSUE 9). (Accessed: 14 August 2025).
McNamara, H.C. et al. (2021) ‘Peripheral, Central, and Cross Sensitization in Endometriosis-Associated Pain and Comorbid Pain Syndromes’, Frontiers in Reproductive Health, 3, p. 729642.
Quintas-Marquès, L. et al. (2023) ‘Central sensitization in patients with deep endometriosis’, Pain Medicine (Malden, Mass.), 24(8), pp. 1005–1007.
Wei, S.-Y. et al. (2016) ‘Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea’, Pain, 157(1), pp. 92–102.
Yam, M.F. et al. (2018) ‘General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation’, International Journal of Molecular Sciences, 19(8), p. 2164.

