Clinical Trials
Our clinical research always builds on years of research by leading neuroscientists, psychiatrists, pain specialists and clinicians, which you are welcome to review on our research page. Previous research had carefully documented the long-term safety and efficacy of neuromodulation on improving low mood and reducing pain symptoms, and research by our collaborators, Prof Rodrigo Pegado (previously - Federal University of Rio Grande do Norte, now - Harvard University) and Prof Maria Micussi (previously - Federal University of Rio Grande do Norte, now - Harvard University) had shown that using menstrual neuromodulation therapy for five days before menstruation reduces pain symptoms in up to 89% of users, and improves PMS symptoms in up to 84% of users with continued and consistent use.
Running clinical trials takes time, so in late 2022 we set up an independent collaboration with Prof Pegado and Prof Micussi to lead our clinical trials investigating the short-term (single period intervention) effects of using Nettle for alleviating both PMS and menstrual pain symptoms in women experiencing menstrual pain (primary dysmenorrhea) and premenstrual syndrome (measured with the PSST scale); and now the results are in.
In our WIND (At-home Treatment of Primary Dysmenorrhea using a Wearable IoT Neuromodulation Device: A Triple-Blind, Randomised Sham-Controlled Trial) study, we focused on 3 core metrics:
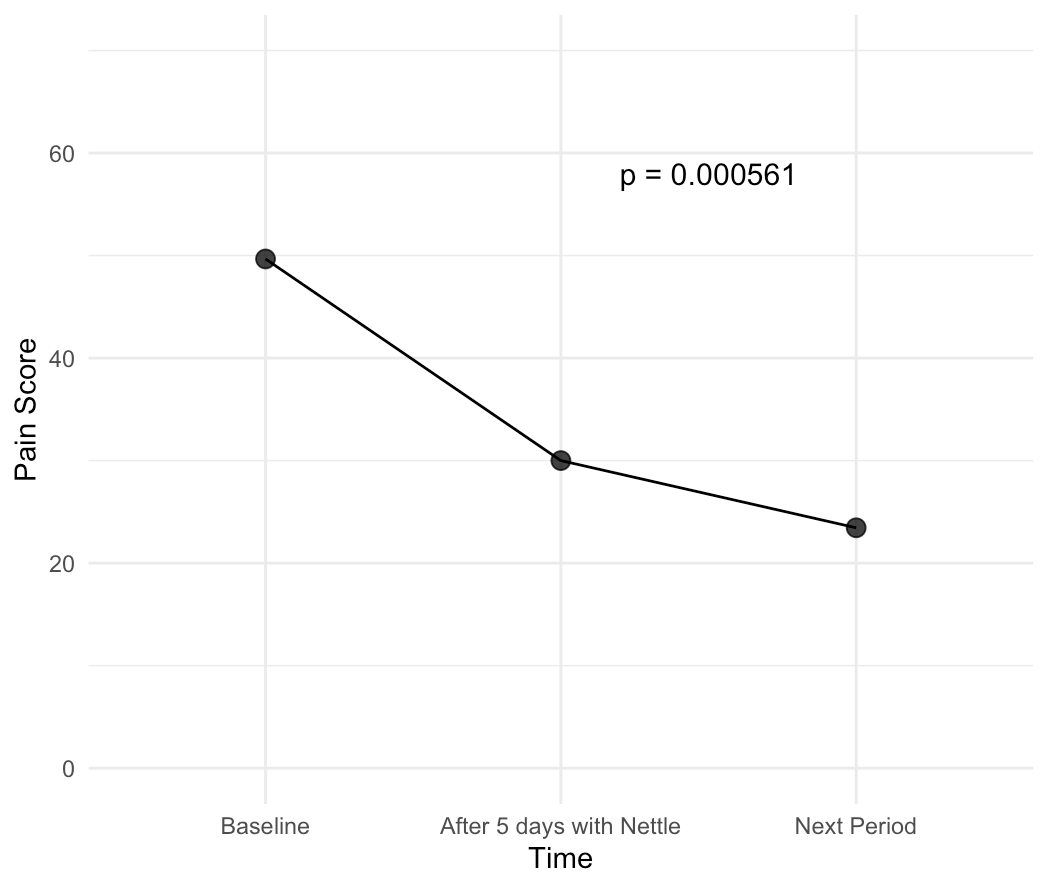
- Reduction of perceived pain, measured using a visual analog scale (score from 0-100) on the most painful day of the menstrual cycle (day 1 or day 2);
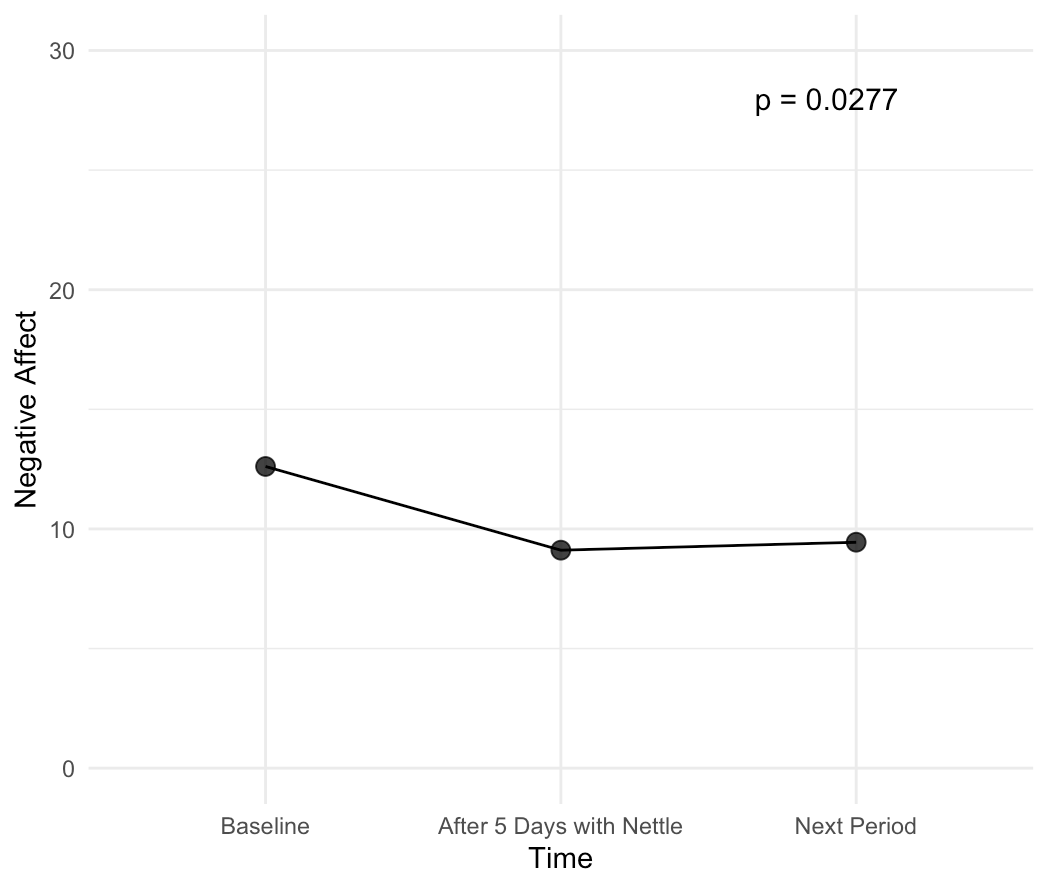
- Improvement in low mood, measured using the PANAS negative scale, which assesses negative affect/emotion;
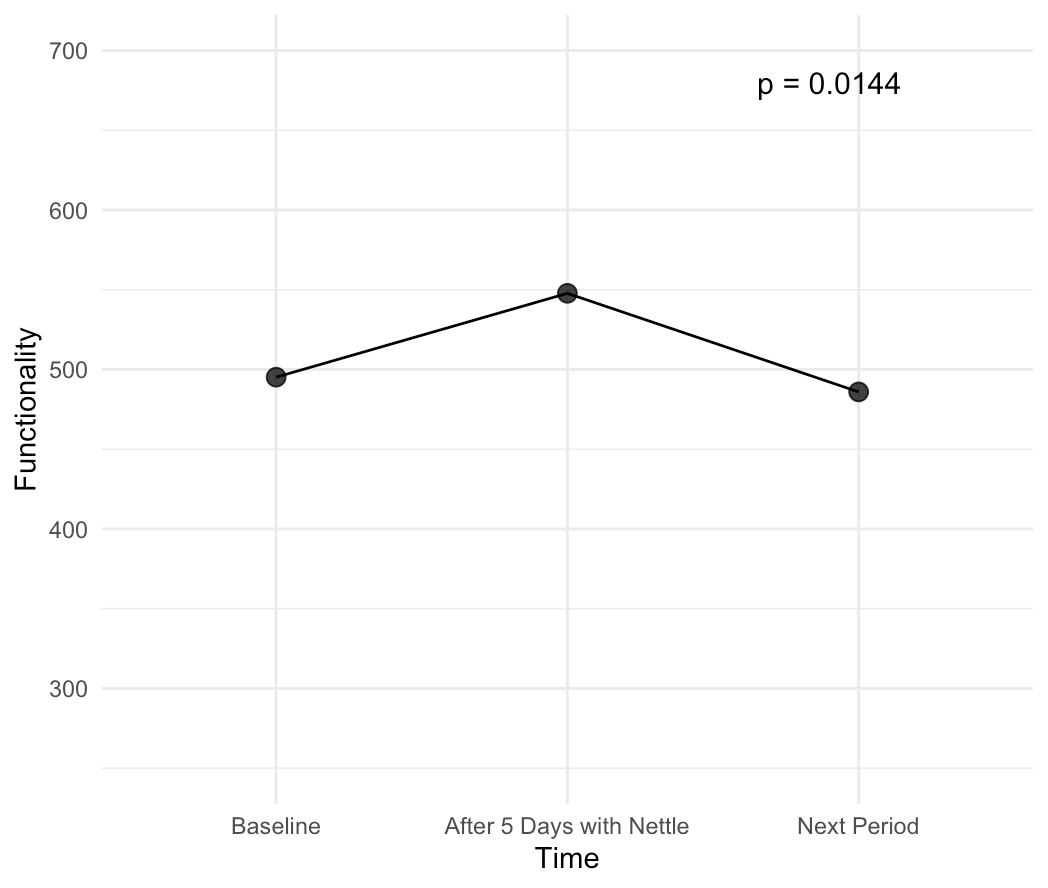
- Improvement in functionality, or the ability to perform everyday tasks, measured using the 6-minute walking test.
We chose these metrics, because our users mentioned that it is the pain, mood and functionality symptoms that impact their wellbeing the most during PMS and menstruation.
We then recruited women with both menstrual pain and PMS symptoms, measured their symptoms for a baseline period, then measured their symptoms after using Nettle daily for 5 days prior to their next period, and then after a month, to see which effects persist due to neuroplasticity and which don’t.
Our study was able to show that, just after a single month's use of Nettle:
These results are incredibly exciting, as they show that Nettle is able to reduce symptoms at least as effectively as painkillers which reduce pain by 45-53% on average, all while having none of the side effects. Additionally, it also is able to improve mood and functionality - key PMS symptoms that currently don’t have medical-grade treatments. This makes us confident in claiming that Nettle truly is the world's first medical grade solution to PMS and menstrual pain.
These results and full methods are available in our preprint here, and have been peer-reviewed and accepted for publication due to come out in mid-July.
Moreover, although Nettle launches this summer, the research does not end here. A crucial part of our mission is to continue investing in research for women's brain and physical health. Below are a few of the clinical trials we are actively participating in and setting up:
- ENHANCE: Endometriosis-Focused Study Helping to Alleviate Chronic Pelvic Pain in Endometriosis Patients (double-blind, sham-controlled, in collaboration with the NHS), June 2024-Jan 2025
- TIARA: Targeting Inter-Hemispheric Alpha Coherence With Nettle To Treat PMDD in at-home settings, July 2023-August 2024
- TETRIS: Targeting Elevated Theta With Nettle To Treat Primary Dysmenorrhea in at-home settings, July 2023-May 2024
We are looking forward to sharing this technology with you when Nettle launches later this year.